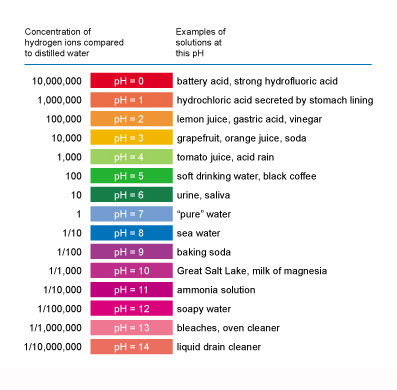
pH Scale
Image courtesy Pacific Institute for the Mathematical Sciences
The ocean is the largest sink for carbon dioxide from human activity, absorbing around 25% of that produced annually. Atmospheric carbon dioxide dissolves into the oceans creating carbonic acid, which has led to an increase in acidity of the ocean surface waters of around 30% since the beginning of the industrial revolution. The fall in ocean surface pH is around 0,002 units per year.
For millions of years, the average pH about the ocean surface was around 8.2 (7.0 is neutral). Today, it is about 8.1. A drop of 0.1 may not seem much, but the pH scale is logarithmic. So a 1-unit drop in pH means a tenfold increase in acidity. A change of 0.1 means a roughly 30% increase in acidity.
The building blocks for the skeletons and shells of many marine organisms are calcium carbonate minerals. The abundance of these minerals will decline as the sea becomes more acidic. This can have dramatic effect on coral reefs and species needing calcifying minerals, including oysters, clams, sea urchins, shallow water corals, deep sea corals, and calcareous plankton.













